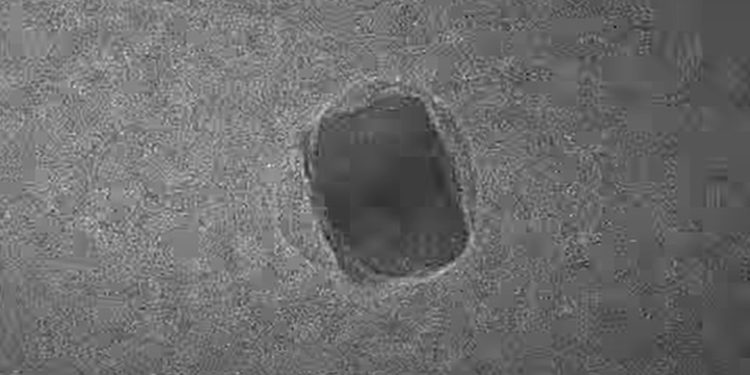
For the first time in history, researchers have visually documented the precise moment when hydrogen and oxygen combine to create water at the nanoscale. This groundbreaking discovery opens new doors in understanding how water is formed at a microscopic level, offering potential applications that stretch far beyond the laboratory.
Earlier this year, a team from Northwestern University, Illinois, developed a cutting-edge technique that allows real-time observation of gas molecules. Using ultra-thin glassy membranes shaped like honeycombs, the scientists managed to trap these molecules within nanoreactors. With the help of high-vacuum transmission electron microscopes, they were able to witness hydrogen and oxygen interact at an atomic scale—a breakthrough for the field.
This advancement allowed the researchers to explore a mystery dating back more than a century: how the rare metal palladium facilitates the rapid creation of water from hydrogen and oxygen. According to Yukun Liu, the study’s lead author, “We’ve known about this phenomenon for years, but understanding it at a molecular level has been elusive. The ability to visualize the water generation process while analyzing atomic structures has enabled us to finally understand what happens during the reaction.”
Witnessing Water Bubbles at the Smallest Scale
During their observations, the team watched in amazement as hydrogen entered the palladium, causing a nanoscale water bubble to form. This discovery marked a significant milestone in the field of nanotechnology.
“We believe this could be the smallest bubble of water ever seen,” said Liu. “It wasn’t what we were expecting, but fortunately, we captured it on video to prove that what we saw was real.”
To deepen their understanding, the researchers employed a specialized technique known as electron energy loss spectroscopy (EELS). This method, previously used to confirm the presence of water on the Moon by India’s Chandrayaan-1 mission, helped them analyze the tiny water bubbles in detail.
Vinayak Dravid, senior author of the study, explained, “By directly visualizing water generation at the nanoscale, we’ve identified optimal conditions for producing water under normal conditions. This has huge potential for practical applications, such as producing water in space using just gases and metal catalysts.”
Implications for Space Exploration and Beyond
The study revealed a surprising discovery: the sequence in which hydrogen and oxygen are introduced to palladium plays a critical role in determining the efficiency of water production. When hydrogen is added before oxygen, the reaction occurs more rapidly, producing water at an accelerated rate. This newfound understanding could dramatically change the way we approach water generation, especially for space missions where resources are limited and efficiency is key.
For instance, astronauts could carry palladium pre-loaded with hydrogen, and by simply introducing oxygen during their mission, they could create a reliable source of drinkable water. This method not only eliminates the need for complex water filtration systems but also offers a more sustainable and efficient way to produce water in the harsh environments of space. This breakthrough has the potential to transform long-term space travel, making it easier to sustain human life on extended missions to other planets or space stations.
As revealed by IFLScience, Dravid drew parallels with Mark Watney, the character from the film The Martian. “In the movie, Watney burns rocket fuel to obtain hydrogen and then adds oxygen to create water. Our process is similar, but far more efficient—we bypass the need for fire or extreme conditions. Instead, we simply mix palladium with the gases.”
This groundbreaking study, featured in the Proceedings of the National Academy of Sciences, introduces groundbreaking possibilities for water generation in the most extreme environments, including the depths of space. As scientists further refine these innovative techniques, their potential could not only help combat water shortages on Earth but also revolutionize how we explore and colonize the solar system.
By pushing the boundaries of our understanding of water at the nanoscale, this discovery marks a pivotal leap in both nanotechnology and chemistry, offering a transformative approach to one of life’s most essential resources: water.